
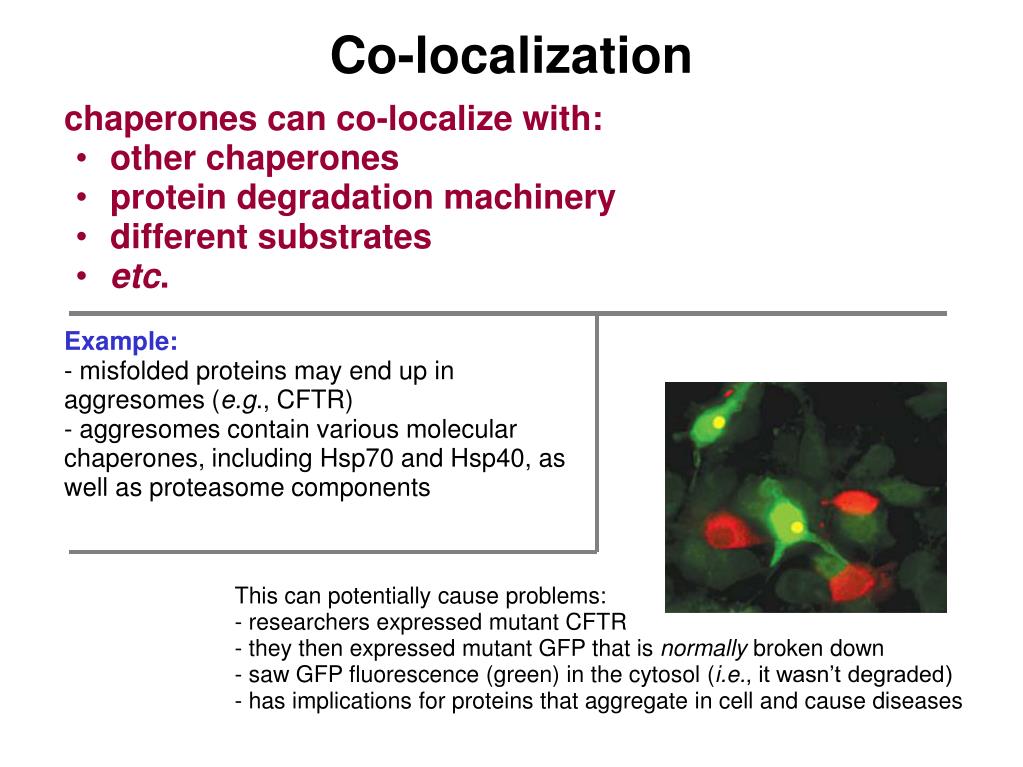
Shows that both TF and Hsp70 (DnaK) bind nascent polypeptides and are crucial for the folding of newly synthesized proteins.īukau, B. Polypeptide flux through bacterial Hsp70: DnaK cooperates with trigger factor in chaperoning nascent chains.
Trigger factor's peptidyl-prolyl isomerase activity is not essential for the folding of cytosolic proteins in Escherichia coli. In vivo analysis of the overlapping functions of DnaK and trigger factor. Dynamic association of trigger factor with protein substrates. Localization of the trigger factor binding site on the ribosomal 50S subunit. The nature of the specific interaction between ribosomes and the nascent-chain-binding chaperone TF is determined.īlaha, G. L23 protein functions as a chaperone docking site on the ribosome. The amino-terminal 118 amino acids of Escherichia coli trigger factor constitute a domain that is necessary and sufficient for binding to ribosomes. Binding specificity of Escherichia coli trigger factor. Escherichia coli trigger factor is a prolyl isomerase that associates with nascent polypeptide chains. Nascent membrane and presecretory proteins synthesized in Escherichia coli associate with signal recognition particle and trigger factor. Cooperation of enzymatic and chaperone functions of trigger factor in the catalysis of protein folding. Scholz, C., Stoller, G., Zarnt, T., Fischer, G. Three-dimensional structures of translating ribosomes by cryo-EM. The structural basis of ribosome activity in peptide bond synthesis. Nissen, P., Hansen, J., Ban, N., Moore, P. Macromolecular crowding: obvious but underappreciated. The fundamentals of protein folding: bringing together theory and experiment. Principles that govern the folding of protein chains. Some eukaryotic polypeptides are passed from the Hsp70 protein HSC70 (70-kDa heat-shock cognate protein) onto chaperones of the Hsp90 (90-kDa heat-shock protein) family, which can work with several co-chaperones to assist the folding of particular proteins.Ĭo-chaperones of HSC70 and HSP90 are also used to sort polypeptides for mitochondrial targeting or to target them for degradation by the proteasome.Īnfinsen, C. The eukaryotic chaperone GimC/prefoldin can bind nascent chains and work with TRiC to fold actin and tubulin. In eukaryotes, Hsp70 chaperones can cooperate with the chaperonin TCP1 ring complex (TRiC) to fold newly synthesized polypeptides both during and after their translation (for example, some WD40-repeat proteins). In Saccharomyces cerevisiae, the ribosome-associated complex recruits the Ssb Hsp70 chaperones to nascent chains.ĭnaK, which is the Escherichia coli Hsp70, works together with the chaperonin GroEL to fold newly synthesized proteins polypeptides can be transferred between these chaperones. To help the folding of nascent polypeptides, the chaperone and peptidyl-prolyl isomerase trigger factor, which is docked on the bacterial ribosome, binds them as they emerge from the ribosomal exit site.Įukaryotic Hsp70 chaperones bind nascent chains as they emerge from ribosomes. The core chaperone machinery - the 70-kDa heat-shock proteins (Hsp70) and chaperonins - is maintained from prokaryotes to eukaryotes, although the chaperone network is expanded in eukaryotes. In the cytosol of prokaryotes and eukaryotes, networks of molecular chaperones can assist polypeptides at all stages of folding.


 0 kommentar(er)
0 kommentar(er)
